China's Push For US Drug Import Substitution
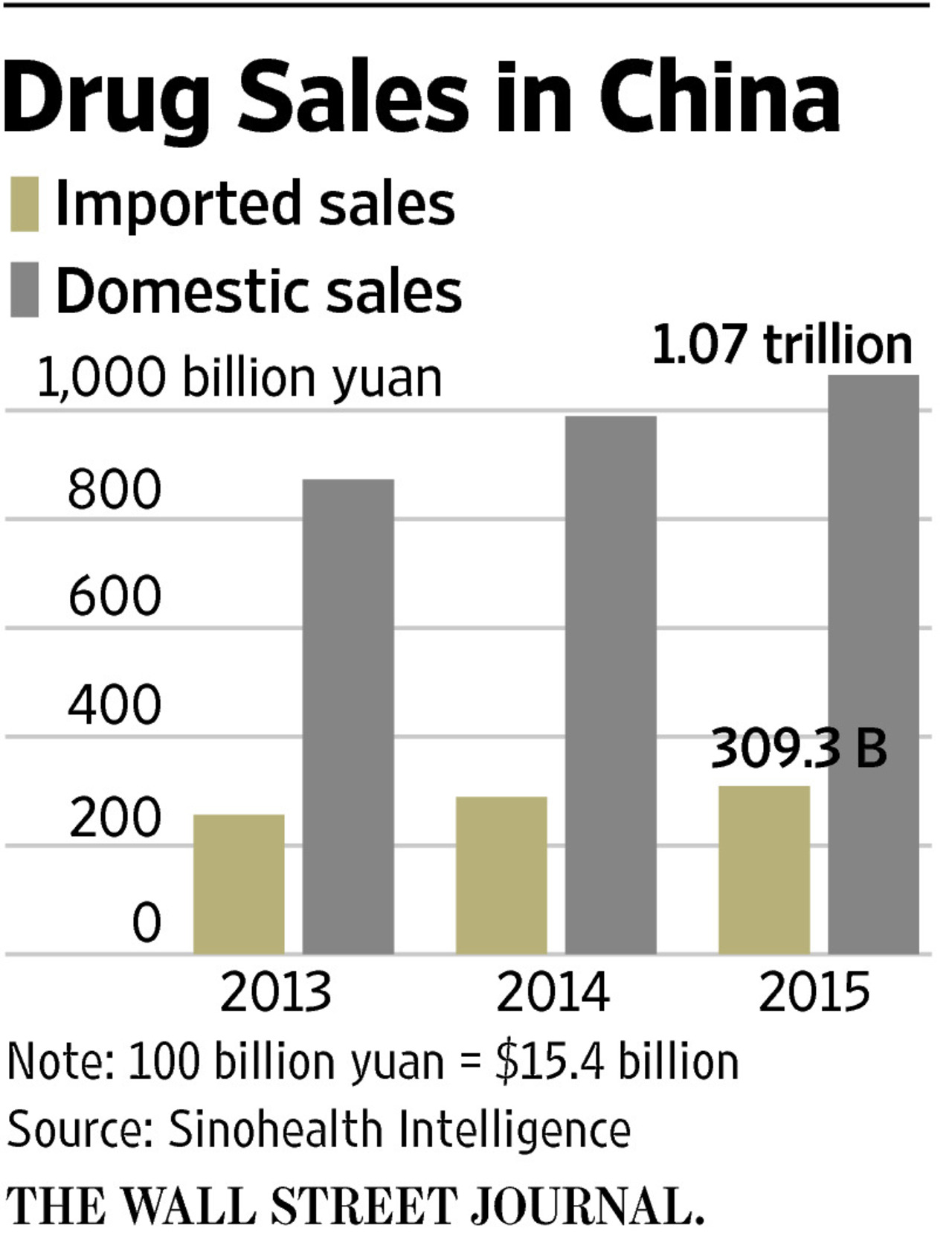
Table of Contents
China's Strategic Goals in Drug Import Substitution
China's ambitions in the pharmaceutical sector extend far beyond mere market share gains. The country's long-term objectives are multifaceted, aiming for self-sufficiency, technological leadership, and ultimately, global pharmaceutical dominance. This ambitious strategy is fueled by significant government investment and proactive policy changes.
- Reducing reliance on foreign drug imports: China aims to decrease its dependence on imported medicines, strengthening its national healthcare security.
- Developing its domestic pharmaceutical manufacturing capabilities: Massive investments are being channeled into expanding manufacturing facilities and upgrading technology within the country.
- Fostering innovation in drug discovery and development: Significant resources are dedicated to research and development, aiming to produce innovative drugs and compete with global pharmaceutical giants.
- Expanding its global market share in pharmaceuticals: Chinese pharmaceutical companies are actively pursuing international expansion, aiming to become major players in global markets.
This initiative is driven by supportive government policies, including generous financial incentives, tax breaks, and streamlined regulatory processes for domestic pharmaceutical companies. We're seeing clear examples of this in the aggressive global expansion of companies like [insert example of a Chinese pharmaceutical company expanding globally].
Impact on US Pharmaceutical Companies
China's push for US drug import substitution poses a significant threat to US pharmaceutical companies. The increased competition from lower-priced Chinese generics and the potential for market share erosion are major concerns.
- Loss of market share in the US and globally: US companies face intense competition, particularly in the generics market, where Chinese manufacturers often offer lower prices.
- Pressure on pricing and profit margins: The influx of affordable Chinese drugs is squeezing profit margins for US companies, necessitating cost-cutting measures and strategic adaptations.
- Increased need for innovation and efficiency: To remain competitive, US companies must prioritize innovation and develop more efficient manufacturing processes.
- Potential for strategic partnerships or acquisitions: Some US companies might explore strategic partnerships or acquisitions of Chinese firms to gain access to the Chinese market and mitigate the competitive threat.
The impact is already visible. [Insert example of a US company impacted by Chinese competition]. US companies need to adapt to this new reality by focusing on innovation in high-value drugs, specialized therapies, and developing strong intellectual property protection strategies.
The Role of Intellectual Property Rights (IPR)
The issue of intellectual property rights (IPR) is central to understanding China's drug import substitution strategy. Concerns regarding intellectual property theft and the enforcement of patent laws remain significant challenges.
- Concerns about intellectual property theft: The history of IP infringement in China raises concerns about the protection of US pharmaceutical innovations.
- Enforcement of patent laws in China: While China has strengthened its IPR laws, enforcement remains a key concern for foreign companies.
- Impact of trade disputes on pharmaceutical trade: Trade disputes between the US and China have further complicated the issue, impacting the flow of pharmaceutical products and technologies.
- The role of international cooperation in addressing IPR concerns: International cooperation is crucial for establishing clear guidelines and effective mechanisms for protecting intellectual property in the pharmaceutical sector.
Numerous examples of IPR disputes between Chinese and US companies exist, highlighting the need for robust international frameworks and stricter enforcement mechanisms. [Insert example of an IPR dispute between a Chinese and US pharmaceutical company].
Economic and Geopolitical Implications
China's push for US drug import substitution has profound economic and geopolitical implications extending beyond the pharmaceutical industry itself.
- Impact on global pharmaceutical pricing: Increased competition from Chinese manufacturers could lead to lower drug prices globally, impacting both healthcare systems and pharmaceutical company profits.
- Shifts in global supply chains: China's growing role in pharmaceutical manufacturing is reshaping global supply chains, potentially increasing reliance on China for crucial medicines.
- Implications for US healthcare costs: Lower drug prices from China could potentially reduce healthcare costs in the US, but also pose risks to domestic pharmaceutical innovation.
- Potential for increased trade tensions between the US and China: The competition for dominance in the pharmaceutical market could exacerbate existing trade tensions between the two countries.
The implications for global healthcare access and affordability are significant. A shift towards more affordable medicines could benefit patients worldwide, but it also raises concerns about the sustainability of pharmaceutical innovation and the potential for disruptions to global supply chains.
Conclusion
China's push for US drug import substitution represents a major shift in the global pharmaceutical landscape. This strategy, driven by ambitious national goals and significant government investment, presents both challenges and opportunities for the US and the global community. The impact on US pharmaceutical companies, the complexities of intellectual property rights, and the broader economic and geopolitical consequences necessitate a careful and strategic response. Understanding China's drug import substitution strategy is crucial for navigating the evolving landscape of the global pharmaceutical market. Stay informed and adapt your strategies accordingly to manage the challenges and capitalize on the opportunities presented by the implications of China's drug market expansion. Ignoring China's growing pharmaceutical influence would be a strategic misstep.

Featured Posts
-
 Eurovision 2025 Basel Confirms Funding For Village
May 01, 2025
Eurovision 2025 Basel Confirms Funding For Village
May 01, 2025 -
 Sheens Million Pound Giveaway Christopher Stevens Channel 4 Review
May 01, 2025
Sheens Million Pound Giveaway Christopher Stevens Channel 4 Review
May 01, 2025 -
 Cardinale Becciu Aggiornamenti Sulla Sua Posizione In Vaticano
May 01, 2025
Cardinale Becciu Aggiornamenti Sulla Sua Posizione In Vaticano
May 01, 2025 -
 Meta And The Ftc Key Developments In The Instagram And Whats App Antitrust Case
May 01, 2025
Meta And The Ftc Key Developments In The Instagram And Whats App Antitrust Case
May 01, 2025 -
 Review Of Splice Cay Fest Through The Lens
May 01, 2025
Review Of Splice Cay Fest Through The Lens
May 01, 2025
