CRISPR Gene Editing: Enhanced Accuracy And Effectiveness Through Novel Modification

Table of Contents
Improving CRISPR Specificity to Reduce Off-Target Effects
One of the major hurdles in utilizing CRISPR-Cas9 is the risk of off-target effects – unintended edits at locations in the genome other than the desired target site. These off-target edits can lead to unpredictable consequences and compromise the safety of gene editing therapies. Minimizing these effects is crucial for translating CRISPR's potential into clinical applications.
Several novel modifications are significantly enhancing CRISPR specificity:
-
Improved guide RNA (gRNA) design: Scientists are developing more sophisticated gRNAs. Truncated gRNAs, for example, exhibit improved specificity by reducing the length of the RNA molecule interacting with the target DNA. Similarly, modifications to the nucleobases within the gRNA can enhance binding affinity to the target sequence, thereby reducing off-target binding.
-
Utilizing Cas variants with enhanced specificity: Engineered Cas9 variants, such as Cas9-HF1 and SpCas9-NG, have been developed with improved target recognition capabilities. These variants demonstrate a significantly reduced rate of off-target cleavages compared to the wild-type Cas9 enzyme. [Cite relevant research paper here, e.g., PMID: 30361668 for Cas9-HF1].
-
Employing paired nickases or base editors for increased precision: Instead of relying on a single Cas9 enzyme to cut both DNA strands, using paired nickases introduces cuts on each strand separately. This approach significantly reduces the chance of off-target events. Base editors further refine this precision by directly altering single bases without causing a double-stranded DNA break. [Cite relevant research paper here, e.g., a review article on base editors].
These modifications dramatically improve target recognition, minimizing unintended edits and greatly increasing the safety and efficacy of CRISPR-based gene editing therapies.
Enhancing CRISPR Delivery Systems for Efficient Gene Editing
Effective gene editing requires efficient delivery of the CRISPR components (Cas enzyme and gRNA) into the target cells or tissues. This presents a significant challenge, as many cells have robust mechanisms to prevent the entry of foreign molecules.
Advancements in delivery methods are addressing this challenge:
-
Viral vectors: Viral vectors, such as adeno-associated viruses (AAVs) and lentiviruses, are widely used for gene delivery due to their ability to efficiently infect various cell types. However, AAVs have limited packaging capacity, while lentiviruses can integrate into the host genome, posing potential risks.
-
Non-viral delivery methods: Non-viral methods, including lipid nanoparticles (LNPs) and electroporation, offer alternatives to viral delivery. LNPs encapsulate the CRISPR components, facilitating cellular uptake, while electroporation uses electric pulses to create temporary pores in the cell membrane, allowing CRISPR components to enter.
-
Targeted delivery systems: Researchers are developing targeted delivery systems to direct CRISPR components specifically to desired cell types or tissues, minimizing off-target effects and maximizing therapeutic efficacy. This often involves combining delivery vectors with specific targeting ligands.
Efficient delivery significantly increases the effectiveness of CRISPR gene editing by ensuring that the editing machinery reaches its intended target. This has a profound impact on therapeutic applications, particularly in cancer therapy (e.g., targeting cancer cells selectively) and the treatment of genetic disorders.
Expanding CRISPR’s Editing Capabilities: Beyond Cas9
While Cas9 is a powerful tool, its limitations—such as its reliance on protospacer adjacent motif (PAM) sequences and its inability to perform certain types of edits— necessitate exploring alternative CRISPR systems.
Several other CRISPR systems offer expanded editing capabilities:
-
Cas12a (Cpf1): Cas12a possesses unique properties, including its smaller size compared to Cas9 and its requirement for a different PAM sequence, enabling targeting of a broader range of genomic sequences.
-
Cas13: Cas13 targets RNA molecules instead of DNA, offering possibilities for gene regulation and treatment of RNA-based diseases.
-
Prime editing: This revolutionary approach allows for precise base-to-base corrections without causing double-stranded DNA breaks, significantly reducing the risk of off-target effects and enabling a wider range of gene edits.
These alternative systems broaden the range of possible gene edits, opening up new avenues for research and therapeutic applications.
Applications of Enhanced CRISPR Technology in Disease Treatment
The improved accuracy and effectiveness of CRISPR technology are already translating into significant advancements in disease treatment:
-
Cancer therapy: CRISPR is enhancing CAR T-cell therapy by improving the specificity and efficiency of targeting cancer cells, leading to more effective treatments with reduced side effects.
-
Genetic disorders: Clinical trials are showing promising results in treating genetic disorders such as sickle cell disease and cystic fibrosis using CRISPR-based therapies.
-
Infectious disease treatment: CRISPR is being explored as a tool for developing novel antiviral therapies, targeting viral genomes and potentially eradicating infections.
Numerous clinical trials are underway, demonstrating the potential of enhanced CRISPR technology for personalized medicine approaches, tailoring treatments to individual patients' genetic profiles.
The Future of Precise Gene Editing with CRISPR
The advancements in improving CRISPR's accuracy and effectiveness are revolutionizing gene therapy and precision medicine. Modifications aimed at increasing specificity, enhancing delivery systems, and expanding editing capabilities are dramatically improving the safety and efficacy of CRISPR-based therapies. Ongoing research is focused on further refining these techniques, exploring novel CRISPR systems, and developing more sophisticated delivery methods. The future holds immense potential for CRISPR gene editing to address a wide range of diseases and revolutionize healthcare. Explore the possibilities of CRISPR gene editing, discover the latest breakthroughs in CRISPR technology, and learn more about the future of CRISPR-based therapies.

Featured Posts
-
 The 30 Minute Glastonbury Ticket Resale Sellout What Happened
May 30, 2025
The 30 Minute Glastonbury Ticket Resale Sellout What Happened
May 30, 2025 -
 Jon Jones Vs Nate Diaz Confirmed Fight Silences Aspinall Rumors
May 30, 2025
Jon Jones Vs Nate Diaz Confirmed Fight Silences Aspinall Rumors
May 30, 2025 -
 Programma Tileoptikon Metadoseon Savvatoy 12 4
May 30, 2025
Programma Tileoptikon Metadoseon Savvatoy 12 4
May 30, 2025 -
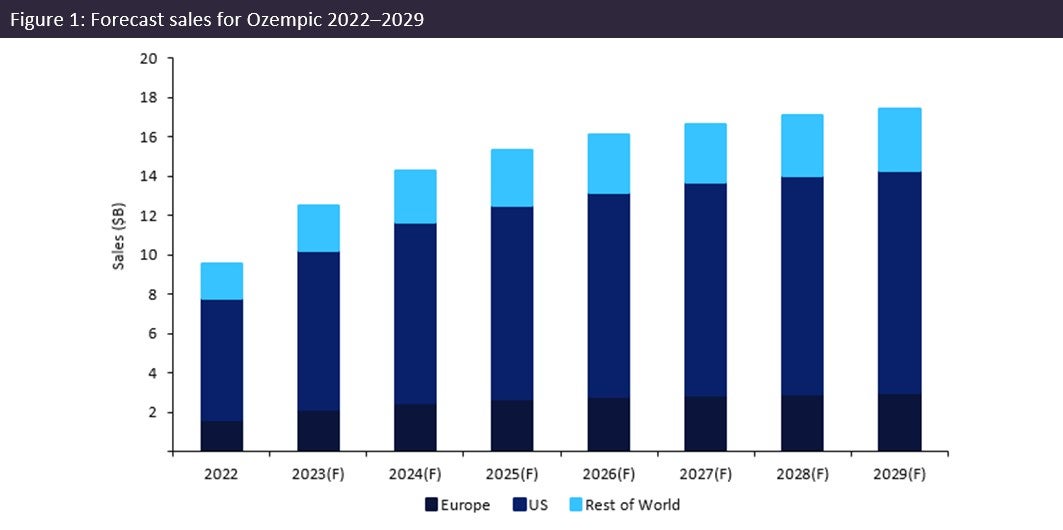 Weight Loss Drugs Novo Nordisks Ozempic And The Competitive Landscape
May 30, 2025
Weight Loss Drugs Novo Nordisks Ozempic And The Competitive Landscape
May 30, 2025 -
 Trumps Trade War 8 Key Impacts On The Canadian Economy
May 30, 2025
Trumps Trade War 8 Key Impacts On The Canadian Economy
May 30, 2025
