Limited Access To Ozempic: The FDA's Response To Counterfeit Products

Table of Contents
The Rise of Counterfeit Ozempic and its Dangers
The high demand for Ozempic, fueled by weight loss trends and its increasing visibility on social media, has created a breeding ground for counterfeit drug production. This presents significant dangers to public health.
Increased Demand Fuels Counterfeit Market
- Weight loss trends: The widespread adoption of Ozempic for weight management has significantly increased demand, exceeding supply in many cases.
- Social media influence: Social media platforms have amplified the perception of Ozempic's efficacy, further driving demand and making it a target for counterfeiters.
- Prescription drug accessibility: While prescription-only, the relative ease of obtaining certain medications online, sometimes through unregulated channels, contributes to the problem.
Taking counterfeit Ozempic can have severe consequences. These fake medications may contain harmful ingredients, incorrect dosages of semaglutide, or no active pharmaceutical ingredient at all. This can lead to ineffective treatment, adverse health effects, and potential long-term complications. The lack of quality control in counterfeit production poses significant risks, making it crucial to source Ozempic only from legitimate pharmacies.
Identifying Counterfeit Ozempic
Identifying fake Ozempic can be challenging, highlighting the need for vigilance. Counterfeiters are becoming increasingly sophisticated in their methods, making visual inspection alone insufficient.
- Visual clues: Look for inconsistencies in printing, unusual coloring, or differences in packaging compared to genuine Ozempic. However, relying solely on visual cues is unreliable.
- Packaging discrepancies: Check for discrepancies in labeling, barcodes, or batch numbers. Pay close attention to details like spelling errors or blurry text.
- Legitimate sources: Only obtain Ozempic from licensed pharmacies, your doctor, or other verified healthcare providers. Never purchase medications from unregulated online sources.
The FDA plays a crucial role in tracking and identifying counterfeit medications. They actively monitor the market, analyzing seized products and collaborating with international agencies to trace the origins of counterfeit drugs. However, the sheer volume of counterfeit products and the sophistication of the criminal networks involved make this a continuous challenge.
The FDA's Response to Counterfeit Ozempic
The FDA has implemented a multi-pronged approach to combat the problem of counterfeit Ozempic and other medications. Their response includes strengthened regulations, public awareness campaigns, and supply chain security improvements.
Enhanced Regulatory Measures
The FDA has taken significant steps to enhance regulations and crack down on those involved in the manufacturing and distribution of counterfeit drugs.
- Increased inspections: The FDA is conducting more frequent inspections of pharmaceutical manufacturing facilities, both domestically and internationally, to ensure compliance with good manufacturing practices.
- Stricter import controls: The agency is tightening controls on the import of pharmaceuticals to prevent counterfeit drugs from entering the country.
- International collaborations: The FDA collaborates with international regulatory agencies and law enforcement to track and disrupt global counterfeit drug networks.
- Criminal prosecutions: The FDA actively pursues criminal prosecutions against individuals and organizations involved in the manufacture and distribution of counterfeit medications.
Regulating the global pharmaceutical market and combating cross-border trafficking of counterfeit drugs is incredibly complex. It requires international cooperation, advanced technologies, and robust regulatory frameworks.
Public Awareness Campaigns
The FDA is actively involved in educating the public about the dangers of counterfeit medications and how to identify and avoid them.
- Public service announcements: Public service announcements (PSAs) are used to raise awareness about the problem of counterfeit drugs and emphasize the importance of obtaining medications from legitimate sources.
- Educational materials: The FDA provides educational materials and resources on its website to help consumers understand how to identify counterfeit medications.
- Partnerships with healthcare providers: The FDA collaborates with healthcare providers and patient advocacy groups to educate patients about medication safety and the dangers of counterfeit Ozempic.
Patient education plays a vital role in preventing the purchase and use of counterfeit medications. Informed patients are less likely to fall victim to fraudulent products.
Supply Chain Security Improvements
The FDA is working to improve the security and transparency of the pharmaceutical supply chain to reduce the availability of counterfeit medications.
- Track-and-trace technologies: The implementation of track-and-trace technologies allows for the monitoring of medications throughout the supply chain, from manufacturing to dispensing.
- Serialization of medications: Serialization involves assigning unique identifiers to individual medication packages, making it easier to track and identify counterfeit products.
- Data-sharing initiatives: The FDA promotes data-sharing initiatives among various stakeholders in the supply chain to enhance transparency and improve detection of counterfeit drugs.
Improved supply chain security offers a powerful defense against counterfeit Ozempic and other medications. By enhancing traceability and transparency, it becomes much harder for counterfeiters to infiltrate the legitimate pharmaceutical supply chain.
Addressing the Ozempic Shortage and Ensuring Patient Access
The high demand for Ozempic, coupled with the challenges of combating counterfeits, has created a complex situation that necessitates a multifaceted approach.
Balancing Supply and Demand
Addressing the Ozempic shortage and ensuring access for legitimate patients requires a comprehensive strategy:
- Increased production capacity: Pharmaceutical manufacturers are working to increase production capacity to meet the growing demand for Ozempic.
- Equitable distribution: Strategies are being developed to ensure fair and equitable distribution of Ozempic to patients who need it.
- Alternative medications: Healthcare providers are exploring alternative medications for patients who cannot access Ozempic due to shortages or counterfeit concerns.
Managing drug shortages and ensuring fair access to essential medications is an ongoing challenge that demands collaborative efforts from manufacturers, regulators, and healthcare providers.
The Role of Healthcare Professionals
Healthcare professionals play a critical role in ensuring patients receive legitimate Ozempic and managing expectations:
- Responsible prescribing practices: Doctors should follow responsible prescribing practices to prevent unnecessary demand and ensure that medications are used appropriately.
- Patient education: Healthcare providers should educate their patients about the dangers of counterfeit medications and the importance of obtaining drugs from legitimate sources.
- Referral to legitimate sources: Doctors should direct patients to reliable pharmacies and other legitimate sources for obtaining their medications.
Open communication between doctors and patients regarding medication safety and the dangers of counterfeit drugs is essential for protecting patient health.
Conclusion
The FDA's response to the problem of counterfeit Ozempic involves a comprehensive strategy encompassing regulatory actions, public awareness campaigns, and supply chain security improvements. Counterfeit medications pose a significant threat to patient safety, and obtaining medication from legitimate sources is crucial. The challenges of balancing supply and demand, addressing shortages, and ensuring patient access to genuine Ozempic require ongoing efforts from various stakeholders.
Call to Action: Learn more about identifying counterfeit Ozempic and protecting yourself from fraudulent medication by visiting the FDA website and consulting your doctor or pharmacist. Always obtain your Ozempic from a legitimate pharmacy or healthcare provider and report any suspected counterfeit medications to the FDA immediately. Stay informed about the latest updates on Ozempic availability and the FDA's ongoing efforts to combat counterfeit drugs.

Featured Posts
-
 This Week In Gbr Grocery Shopping Guide 2 000 Quarter Found And Doge Poll Update
May 22, 2025
This Week In Gbr Grocery Shopping Guide 2 000 Quarter Found And Doge Poll Update
May 22, 2025 -
 Peppa Pigs Big Screen Adventure 10 Episodes This May
May 22, 2025
Peppa Pigs Big Screen Adventure 10 Episodes This May
May 22, 2025 -
 European Union Trade Policy Macron Advocates For Domestic Goods
May 22, 2025
European Union Trade Policy Macron Advocates For Domestic Goods
May 22, 2025 -
 Abn Amro Facing Dutch Central Bank Scrutiny Over Bonus Payments
May 22, 2025
Abn Amro Facing Dutch Central Bank Scrutiny Over Bonus Payments
May 22, 2025 -
 We Tested Googles Ai Smart Glasses Prototype Heres What We Found
May 22, 2025
We Tested Googles Ai Smart Glasses Prototype Heres What We Found
May 22, 2025
Latest Posts
-
 Cac Tuyen Duong Ket Noi Tp Hcm Va Ba Ria Vung Tau Huong Dan Chi Tiet
May 22, 2025
Cac Tuyen Duong Ket Noi Tp Hcm Va Ba Ria Vung Tau Huong Dan Chi Tiet
May 22, 2025 -
 Kham Pha Mang Luoi Giao Thong Tp Hcm Ba Ria Vung Tau
May 22, 2025
Kham Pha Mang Luoi Giao Thong Tp Hcm Ba Ria Vung Tau
May 22, 2025 -
 Du An Cau Ma Da Dong Nai Binh Phuoc Duoc Ket Noi Khoi Cong Thang 6
May 22, 2025
Du An Cau Ma Da Dong Nai Binh Phuoc Duoc Ket Noi Khoi Cong Thang 6
May 22, 2025 -
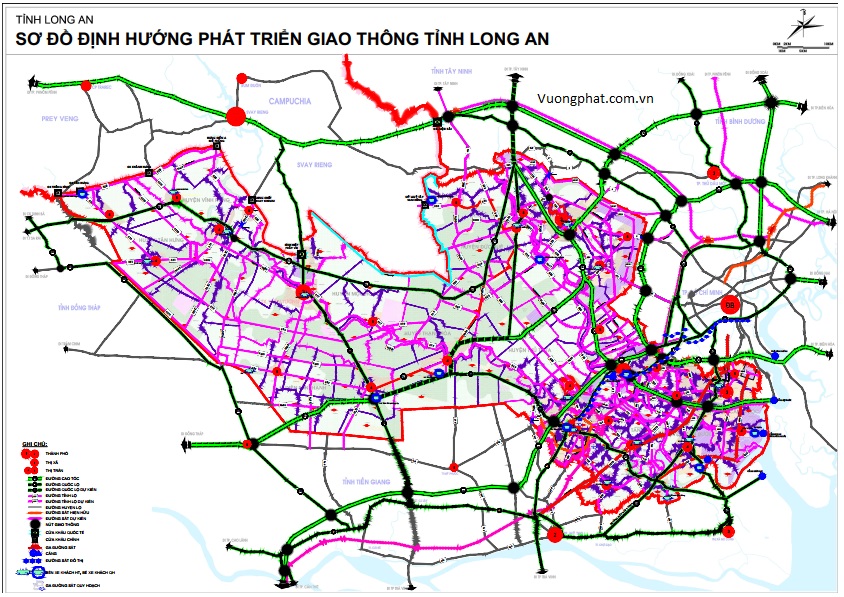 Chien Luoc Phat Trien 7 Tuyen Giao Thong Chinh Tp Hcm Long An
May 22, 2025
Chien Luoc Phat Trien 7 Tuyen Giao Thong Chinh Tp Hcm Long An
May 22, 2025 -
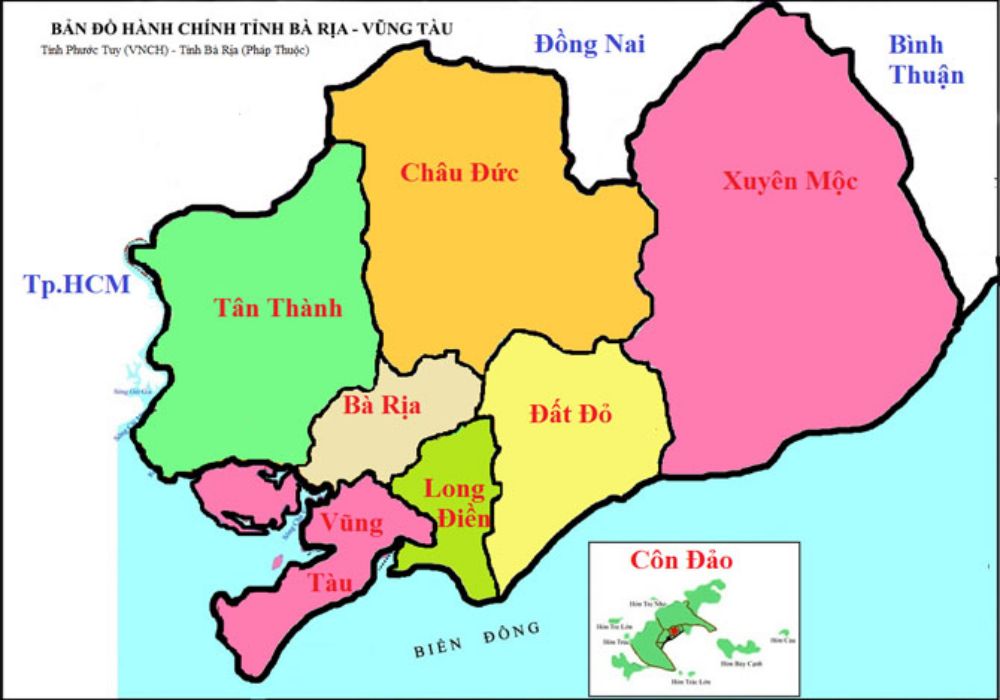 Giai Doan Ket Noi Giao Thong Tp Hcm Ba Ria Vung Tau Hien Trang Va Tuong Lai
May 22, 2025
Giai Doan Ket Noi Giao Thong Tp Hcm Ba Ria Vung Tau Hien Trang Va Tuong Lai
May 22, 2025
