Urgent Recall Notice: Check Your Dressings And Birth Control Pills (Ontario & Canada)
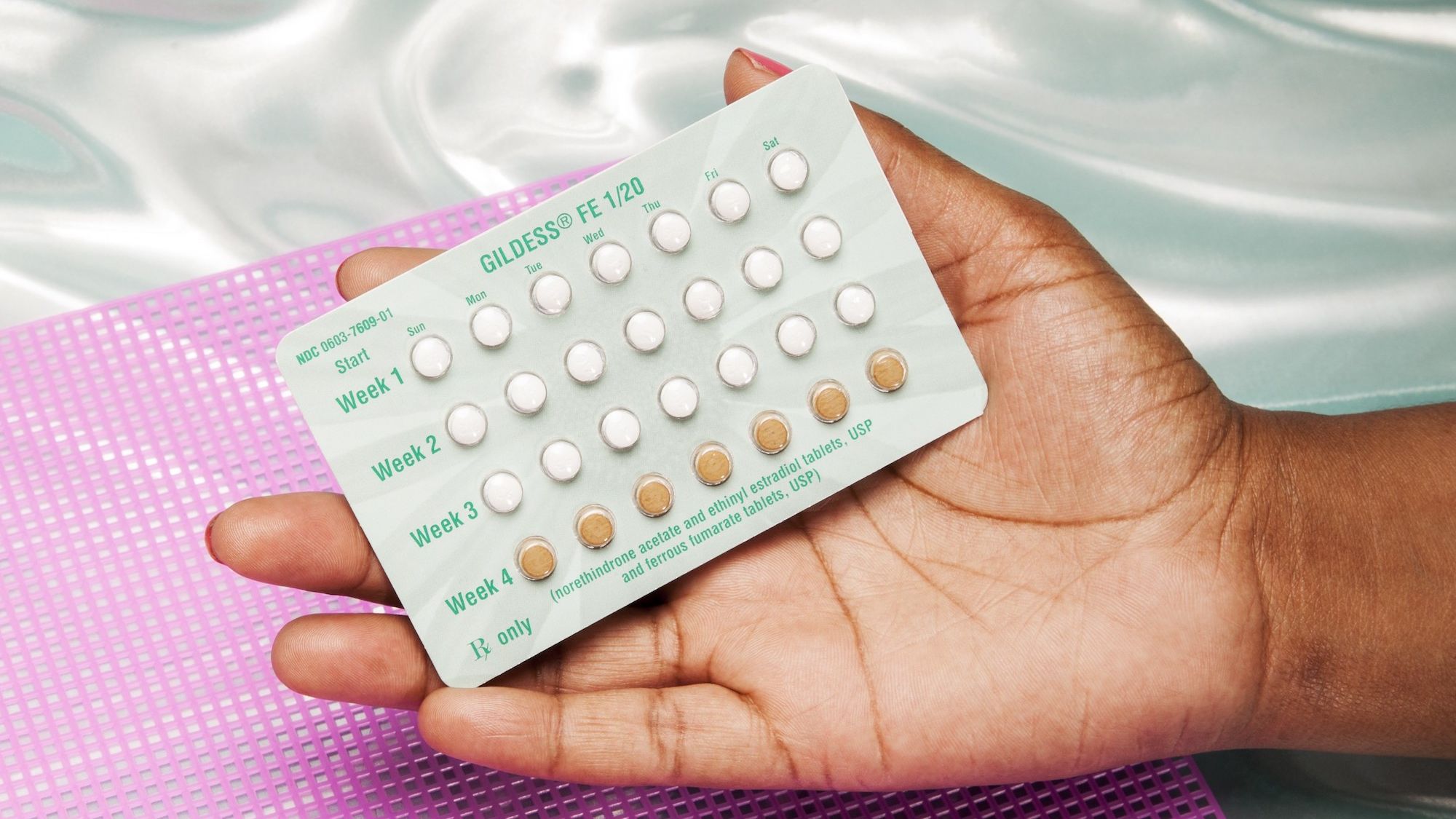
Table of Contents
A critical recall has been issued across Ontario and Canada impacting certain medical dressings and birth control pills. This urgent notice details the affected products, why they've been recalled, and what actions you should take to ensure your safety and well-being. Knowing how to identify these recalled items is crucial. This information is vital for your health and safety, so please read carefully.
Recalled Dressings: Identification and Risks
Identifying Recalled Dressings:
This recall affects specific batches of several brands of wound dressings. It is critical to check your supplies immediately. To help you identify the recalled products, we've provided the following information:
- Brand A: Product Code: AB1234, Expiry Date: 2024-03-15. (Image of packaging would be inserted here) Characteristics: White, non-woven material, rectangular shape.
- Brand B: Product Code: BC5678, Expiry Dates: between 2024-04-20 and 2024-05-10. (Image of packaging would be inserted here) Characteristics: Beige gauze, square shape.
- Brand C: Product Code: CD9012, Expiry Date: 2024-06-30. (Image of packaging would be inserted here) Characteristics: Sterile, individually wrapped, circular shape.
Potential Risks Associated with Recalled Dressings:
The recalled dressings have the potential to cause serious health complications. The main risks identified include:
- Infection: Contamination may lead to bacterial or fungal infections at the wound site, potentially requiring further medical treatment.
- Allergic Reactions: Some individuals may experience allergic reactions, such as rash, itching, or swelling, due to the presence of an undisclosed allergen.
- Impaired Wound Healing: The compromised integrity of the dressing may hinder proper wound healing, prolonging recovery time.
One reported incident involved a severe infection requiring hospitalization. This underlines the urgency of this recall.
Actions to Take if You Have Recalled Dressings:
If you possess any of the dressings listed above, take the following steps immediately:
- Stop using the product immediately. Do not apply the dressing to any wound.
- Dispose of the recalled dressings properly: Seal them in a plastic bag and discard them in your household garbage.
- Contact the manufacturer: For details on potential reimbursement, contact the manufacturer using the contact information provided below.
- Seek medical attention: If you have experienced any adverse reactions after using these dressings, consult a doctor or visit your nearest urgent care center immediately.
Recalled Birth Control Pills: Identification and Risks
Identifying Recalled Birth Control Pills:
The following birth control pills have been recalled due to potential manufacturing defects:
- Brand X: Dosage: 20mcg Ethinylestradiol / 1mg Levonorgestrel, Lot Numbers: XY123, XY456. (Image of packaging would be inserted here)
- Brand Y: Dosage: 30mcg Ethinylestradiol / 150mcg Desogestrel, Lot Number: YZ789. (Image of packaging would be inserted here)
Carefully check the brand name, dosage, and lot number on your pill pack.
Potential Risks Associated with Recalled Birth Control Pills:
Taking the recalled birth control pills may lead to:
- Unintended Pregnancy: The potential for reduced effectiveness means there's a higher chance of unintended pregnancy.
- Hormonal Imbalances: Irregular bleeding, mood changes, and other hormonal imbalances are possible.
While no adverse effects have been officially reported yet, the risk is significant enough to warrant immediate action.
Actions to Take if You Have Recalled Birth Control Pills:
If you are taking the recalled birth control pills:
- Contact your doctor or pharmacist immediately: Discuss alternative birth control options and any potential health concerns.
- Do not stop taking your pills abruptly: Consult your doctor for guidance on safely transitioning to another method.
- Consider additional contraceptive methods: Until you receive further guidance from your doctor, use alternative forms of birth control to prevent pregnancy.
Where to Find More Information:
Official Government Resources:
- Health Canada: [Insert Health Canada Website Link Here]
- Ontario Ministry of Health: [Insert Ontario Ministry of Health Website Link Here]
Contacting Manufacturers:
- Brand A: [Insert Contact Information Here]
- Brand B: [Insert Contact Information Here]
- Brand C: [Insert Contact Information Here]
- Brand X: [Insert Contact Information Here]
- Brand Y: [Insert Contact Information Here]
Conclusion:
This recall affects crucial medications and medical supplies. It's vital to check your medicine cabinet and first-aid kit immediately for the recalled dressings and birth control pills. If you find any of the affected products, follow the instructions provided in this article. Contacting healthcare professionals for advice is crucial. Don't delay—your health and safety are paramount. Staying informed about drug recalls and product recalls is vital for protecting your well-being. Check your supplies now!

Featured Posts
-
 Epl Nottingham Forest Offer Update On Awoniyis Fitness
May 14, 2025
Epl Nottingham Forest Offer Update On Awoniyis Fitness
May 14, 2025 -
 La Advertencia Del Sociologo Danny Shaw Sobre La Crisis En Haiti
May 14, 2025
La Advertencia Del Sociologo Danny Shaw Sobre La Crisis En Haiti
May 14, 2025 -
 Cannonball U Tv Show Episode Guide And Schedule
May 14, 2025
Cannonball U Tv Show Episode Guide And Schedule
May 14, 2025 -
 Nottingham Forests Striker Problem Addressing The Deficiency In The Transfer Window
May 14, 2025
Nottingham Forests Striker Problem Addressing The Deficiency In The Transfer Window
May 14, 2025 -
 Maya Jama And Ruben Dias Relationship Confirmed
May 14, 2025
Maya Jama And Ruben Dias Relationship Confirmed
May 14, 2025
